Christmas 2025 update: Two years later
Two years ago, I had no idea that a single veterinary sedative would cost me my best friend and launch me into an advocacy journey I never expected.
Today, on Christmas Day 2025, I’m still here. Still documenting. Still receiving submissions. Still fighting.
The Stories Keep Coming
Even after I made it clear there is no lawsuit. Even after I explained we are documenting a pattern, and anything else we can document that the FDA doesn’t.
The submissions haven’t stopped.
This morning, Christmas morning, I received another submission. A black cat. Given Zorbium® without the owner’s knowledge or consent. Severe adverse reactions. Drooling that never stopped. A year later, euthanized due to pancreatitis that went undiagnosed because the owner was too traumatized the Zorbium® experience to return to the vet.
In fact this very same thing has happened to me.
What We’ve Learned This Year
The data continues to reveal patterns that should concern every pet owner:
- 114 cases submitted and analyzed
- 326 deaths documented in FDA adverse event reports (through 36 months of data analysis)
- Black cats show disproportionately higher adverse reaction rates
- 98.2% of pet owners were never told their cat would receive Zorbium®
That last statistic deserves its own examination. I’ve created a dedicated page about the informed consent crisis because this pattern is so universal it can’t be ignored.
New Patterns Emerging
Today’s submission revealed something I hadn’t fully documented before: the long-term harm caused when Zorbium®’s severe side effects scare pet owners away from veterinary care entirely.
This cat didn’t just die from Zorbium®’s direct toxicity. The cat died because:
“That was the issue all along, and I could have found out sooner if the Zorbium® didn’t scare me off taking him to the vet.”
Healthcare avoidance. Delayed diagnosis. Preventable death from a treatable condition. These are harms that don’t show up in FDA adverse event reports, but they’re just as real and just as devastating.
Why I Keep Going
People ask me why I’m still doing this. Two years later. On Christmas Day. When there’s no lawsuit, no regulatory action, no recall.
Because the stories keep coming. People keep thanking me.
Because every submission represents a pet owner who thought they were alone. Who thought it was their fault. Who thought their vet would never give their cat something dangerous without telling them.
Because 98.2% of pet owners had no idea what was happening to their cats.
Because black cats keep dying at rates that suggest something is very, very wrong.
Because regulatory capture is real – the person now heading the FDA’s Center for Veterinary Medicine is a former Elanco executive, and Elanco manufactures Zorbium®.
Because someone needs to document this pattern, even if all I can do is collect the data and make it available to pet owners who deserve to know what they’re agreeing to when they sign a consent form that says “sedation.”
What’s Next
The work continues:
- Analyzing new submissions as they arrive
- Documenting emerging patterns like healthcare avoidance and delayed diagnosis
- Maintaining the most comprehensive public database of Zorbium® adverse events
- Providing information so pet owners can make informed decisions
- Pushing for transparency and informed consent in veterinary medicine
I don’t take days off from this work because the adverse reactions don’t take days off. The deaths don’t pause for holidays. The pet owners who discover this website on Christmas Day because their cat is dying and they’re desperately searching for answers don’t get to wait until after the holidays to find information.
So I’m here. Still documenting. Still analyzing. Still fighting for the informed consent and transparency that Spooky deserved and didn’t get.
In Memory
Two years without my boy. Two years of turning grief into action. Two years of making sure other pet owners have the information I wish I’d had.
Spooky’s death wasn’t meaningless. Every submission I receive, every data point I document, every pet owner who reads this website and asks their vet questions – that’s his legacy.
Merry Christmas, Spooky. We’re still fighting.
Updated 10/23/2025: Following over 100 submissions from cat and dog parents, we have identified a concerning pattern: adverse reactions to Zorbium® appear to correlate with coat color, with black cats and black-and-white cats experiencing disproportionately higher rates of severe adverse events and fatalities.
We are actively investigating this pattern and will be contacting veterinary pharmacology experts, feline genetics researchers, and academic institutions to further this research. Our goal is to determine whether genetic factors associated with coat pigmentation may influence drug metabolism and response.
If you have experienced an adverse event with Zorbium® in a cat of any coat color, please continue to submit your report. Your data is critical to understanding this potential safety concern.
Updates will be posted as our research progresses.
These graphs represent the experiences cat owners had after Zorbium® was applied to their cat and subsequently filled out our survey. While we know that each case is unique and every loss is deeply personal, visualizing the data in this way helps us understand the broader trends and patterns emerging around this medication.
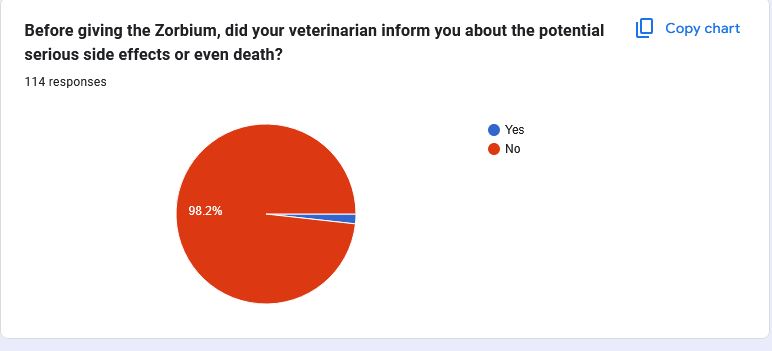
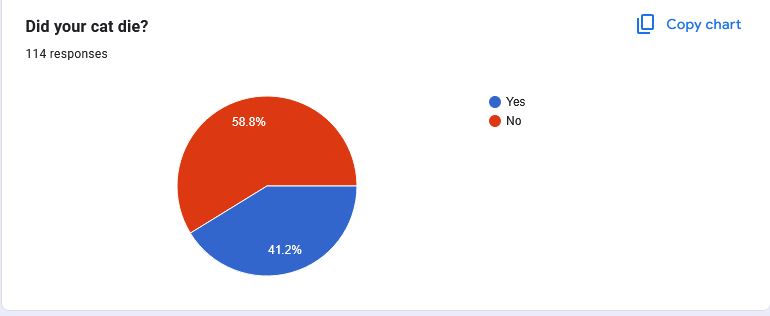
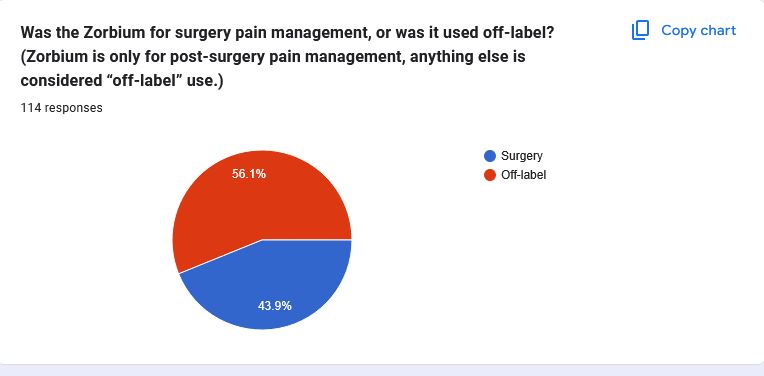
As we continue to gather more information, it’s crucial to note that these results are ongoing and subject to change as more pet owners come forward with their experiences.
Side Effects
From Elanco’s Website 8/6/24
ZORBIUM contains buprenorphine, an opioid that exposes humans to risks of misuse, abuse and addiction, which can lead to overdose and death. Use of buprenorphine may lead to physical dependence. The risk of abuse humans should be considered when storing, administering and disposing of ZORBIUM. Serious, life-threatening or fatal respiratory depression may occur with accidental exposure to or with misuse or abuse of ZORBIUM. ZORBIUM should only be administered through veterinarians or veterinarian technicians who are trained in the handling of potent opioids. Accidental exposure to even one tube of ZORBIUM, especially in children, can result in a fatal overdose.
ZORBIUM is for topical application in cats only. Do not come into direct contact with ZORBIUM. Wear impermeable latex or nitrile gloves, protective glasses and a laboratory coat when applying ZORBIUM. Following application to the cat, allow a minimum drying time of 30 minutes before direct contact with the application site. Do not administer to cats with a known hypersensitivity to buprenorphine hydrochloride, any inactive ingredients of ZORBIUM, or known intolerance to opioids. Do not apply ZORBIUM if the application site at the dorsal cervical area has diseased or injured skin, or to anatomic areas other than the dorsal cervical area because absorption characteristics may be different. Body temperature should be monitored postoperatively. Most common adverse reactions during anesthesia were hypothermia, hypotension and hypertension, and after anesthetic recovery were hypothermia, hyperthermia and sedation. The safe use of ZORBIUM has not been evaluated in debilitated cats; those with renal, hepatic, cardiac or respiratory disease; pregnant, lactating or breeding cats; in cats younger than four months old; or in cats <2.6 lbs. or >16.5 lbs.
The following is a list of side effects reported to the FDA in association with Zorbium use in cats. This information is provided for educational purposes and should not be considered a substitute for professional veterinary advice.
Reported Adverse Reactions to Zorbium
This list was updated 10/13/2024. Data provided through the U.S. Food and Drug Administration (https://open.fda.gov). For the most current information, please consult the FDA’s official resources.
Behavioral and Neurological
- Behavioral disorder NOS (Not Otherwise Specified)
- Lethargy
- Dysphoria
- Hiding
- Hyperactivity
- Restlessness
- Agitation
- Disorientation
- Ataxia
- Sedation
- Seizures
- Mental impairment
- Circling
- Anxiety
- Excitation
- Loss of consciousness
- Confusion
- Twitching
- Pacing
- Staring
- Vocalization
- Hallucination
Cardiovascular and Respiratory
- Hypotension
- Bradycardia
- Tachycardia
- Respiratory distress
- Cyanosis
- Collapse
- Increased heart rate
- Open mouth breathing
- Heavy breathing
- Panting
- Increased respiratory rate
- Hypertension
- Arrhythmia
- Pleural effusion
- Pulmonary oedema
Gastrointestinal
- Anorexia (not eating)
- Constipation
- Vomiting
- Diarrhea
- Difficulty swallowing
- Decreased appetite
- Nausea
- Hypersalivation
- Drooling
Temperature Regulation
- Hyperthermia (high body temperature)
- Hypothermia (low body temperature)
- Fever
- Elevated temperature
Ocular
- Dilated pupils (mydriasis)
- Fixed pupil
- Blindness
- Abnormal pupil light reflex
- Eye irritation
- Squinting
- Third eyelid protrusion
- Anisocoria (unequal pupil size)
Urinary and Renal
- Urinary retention
- Difficulty in urination
- Inappropriate urination
- Kidney disorders
- Not urinating
- Elevated blood urea nitrogen (BUN)
- Elevated creatinine
- Renal failure
Musculoskeletal and Mobility
- Weakness
- Unable to stand
- Stumbling gait
- Unsteady gait
- Falling
- Unable to jump
- Stiffness in limbs
- Muscle wasting
Other Physical Symptoms
- Dehydration
- Pale mucous membranes
- Trembling/Shaking
- Hypersalivation
- Facial swelling
- Weight loss
- Increased sweating
- Pain
- Malaise
- Lethargy
- Inappetence
- Not drinking
- Tongue protrusion
Metabolic and Laboratory Abnormalities
- Hyperglycemia
- Hypoglycemia
- Elevated liver enzymes
- Hypoalbuminemia
- Electrolyte imbalances
- Leucocytosis
- Neutropenia
Severe Outcomes
- Death
- Death euthanasia
- Coma
- Cardiac arrest
Application Site Reactions
- Application site alopecia (hair loss)
- Application site irritation
- Application site redness
- Application site scab
- Application site swelling
- Application site pruritus (itching)
- Application site inflammation
Note: This list is not exhaustive and may not represent all possible adverse reactions. If you notice any unusual symptoms in your pet after administering Zorbium, please contact Elanco immediately. Vets are NOT required to report adverse events.
It’s important to stay informed about the latest official statements and guidelines regarding Zorbium. We encourage all cat owners to consult this FDA information and discuss it with their veterinarians.
Our Commitment
We remain committed to raising awareness, seeking answers, and pushing for change. While we are still unsure if this will become a class action case, we continue to gather information and investigate all possible avenues for justice.
Call for Support
We are actively seeking additional lawyers with experience in animal medication cases to join our cause. If you are a lawyer or know of one who might be interested in contributing to this important work, please contact us.
We are also reaching out to journalists who can help bring more attention to this issue. If you have media contacts or are a journalist interested in covering this story, we would greatly appreciate your support in spreading awareness.